Recombinant Pig Heat shock protein HSP 90-alpha (HSP90AA1), partial
In Stock Promotion-
中文名稱:斯克羅法HSP90AA1重組蛋白
-
貨號:CSB-BP010802PI
-
規(guī)格:¥2832
-
促銷:
-
圖片:
-
其他:
產品詳情
-
純度:Greater than 90% as determined by SDS-PAGE.
-
基因名:
-
Uniprot No.:
-
別名:HSP90AA1; HSP90A; HSPCAHeat shock protein HSP 90-alpha
-
種屬:Sus scrofa (Pig)
-
蛋白長度:Partial
-
來源:Baculovirus
-
分子量:19.7kDa
-
表達區(qū)域:222-367aa
-
氨基酸序列VEKERDKEVSDDEAEEKEDKEEEKEKEEKESEDKPEIEDVGSDEEEEEKKDGDKKKKKKIKEKYIDQEELNKTKPIWTRNPDDITNEEYGEFYKSLTNDWEDHLAVKHFSVEGQLEFRALLFVPRRAPFDLFENRKKKNNIKLYVR
Note: The complete sequence including tag sequence, target protein sequence and linker sequence could be provided upon request. -
蛋白標簽:N-terminal 6xHis-tagged
-
產品提供形式:Liquid or Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
緩沖液:Tris-based buffer,50% glycerol
-
儲存條件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保質期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
貨期:3-7 business days
-
注意事項:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet & COA:Please contact us to get it.
相關產品
靶點詳情
-
功能:Molecular chaperone that promotes the maturation, structural maintenance and proper regulation of specific target proteins involved for instance in cell cycle control and signal transduction. Undergoes a functional cycle that is linked to its ATPase activity which is essential for its chaperone activity. This cycle probably induces conformational changes in the client proteins, thereby causing their activation. Interacts dynamically with various co-chaperones that modulate its substrate recognition, ATPase cycle and chaperone function. Engages with a range of client protein classes via its interaction with various co-chaperone proteins or complexes, that act as adapters, simultaneously able to interact with the specific client and the central chaperone itself. Recruitment of ATP and co-chaperone followed by client protein forms a functional chaperone. After the completion of the chaperoning process, properly folded client protein and co-chaperone leave HSP90 in an ADP-bound partially open conformation and finally, ADP is released from HSP90 which acquires an open conformation for the next cycle. Plays a critical role in mitochondrial import, delivers preproteins to the mitochondrial import receptor TOMM70. Apart from its chaperone activity, it also plays a role in the regulation of the transcription machinery. HSP90 and its co-chaperones modulate transcription at least at three different levels. In the first place, they alter the steady-state levels of certain transcription factors in response to various physiological cues. Second, they modulate the activity of certain epigenetic modifiers, such as histone deacetylases or DNA methyl transferases, and thereby respond to the change in the environment. Third, they participate in the eviction of histones from the promoter region of certain genes and thereby turn on gene expression. Binds bacterial lipopolysaccharide (LPS) and mediates LPS-induced inflammatory response, including TNF secretion by monocytes. Antagonizes STUB1-mediated inhibition of TGF-beta signaling via inhibition of STUB1-mediated SMAD3 ubiquitination and degradation. Mediates the association of TOMM70 with IRF3 or TBK1 in mitochodria outer membrane which promotes host antiviral response.
-
- This study firstly found that host chaperone heat shock protein 90 (Hsp90) had a positive regulatory effect on the porcine circovirus type 2 infection cycle in vitro. PMID: 27553861
- Feed intake remains low whereas respiratory frequency and body temperature remain higher and expression of HSP90, CAT1, SGLT1 and GLUT4 increases in some tissues in pigs under chronic heat stress conditions. PMID: 27264891
- HSP90AA1 sperm content and the prediction of the boar ejaculate freezability. PMID: 20580074
- Hsp90 is a modulator of eNOS activity and vascular reactivity in the newborn piglet pulmonary circulation PMID: 17337508
- Apo-Hsp90 is in a conformational equilibrium between two open states that have never been described previously. PMID: 18215117
- Findings indicate an essential role for Hsp90 in nongenomic estrogen signaling in coronary artery smooth muscle cells. PMID: 19293389
顯示更多
收起更多
-
亞細胞定位:Nucleus. Cytoplasm. Melanosome. Cell membrane. Mitochondrion.
-
蛋白家族:Heat shock protein 90 family
-
組織特異性:Preferentially expressed in pituitary gland, brain, adrenal gland, and testis, in comparison to other tissues.
-
數(shù)據(jù)庫鏈接:
Most popular with customers
-
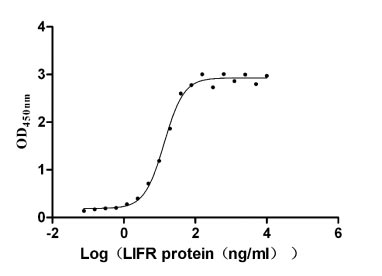
Recombinant Human Leukemia inhibitory factor receptor (LIFR), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Poliovirus receptor (PVR) (I340M), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human E3 ubiquitin-protein ligase ZNRF3 (ZNRF3), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Cannabinoid receptor 1 (CNR1)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Claudin-3 (CLDN3)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Alkaline phosphatase, germ cell type (ALPG) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Rat Gastric inhibitory polypeptide receptor (Gipr), partial (Active)
Express system: Mammalian cell
Species: Rattus norvegicus (Rat)
-
Recombinant Human Cytotoxic and regulatory T-cell molecule (CRTAM), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)




-AC1.jpg)