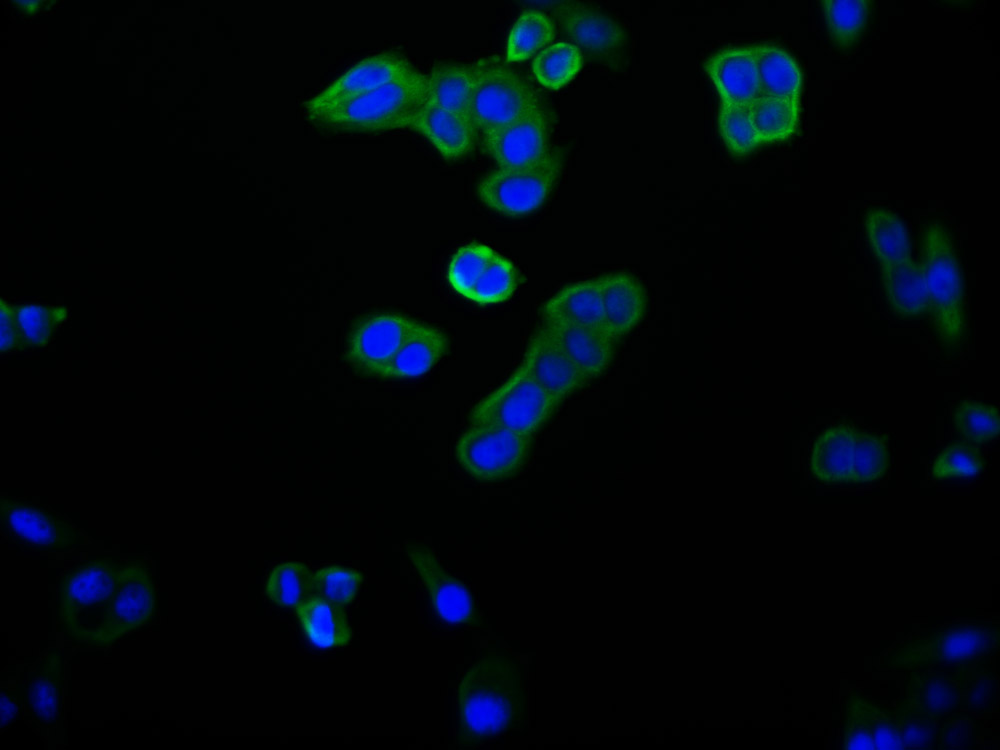
NHLRC1 Antibody, Biotin conjugated
-
中文名稱:NHLRC1兔多克隆抗體, Biotin偶聯(lián)
-
貨號:CSB-PA740936LD01HU
-
規(guī)格:¥880
-
其他:
產(chǎn)品詳情
-
產(chǎn)品名稱:Rabbit anti-Homo sapiens (Human) NHLRC1 Polyclonal antibody
-
Uniprot No.:
-
基因名:
-
別名:E3 ubiquitin-protein ligase NHLRC1 (EC 2.3.2.27) (Malin) (NHL repeat-containing protein 1) (RING-type E3 ubiquitin transferase NHLRC1), NHLRC1, EPM2B
-
宿主:Rabbit
-
反應(yīng)種屬:Human
-
免疫原:Recombinant Human E3 ubiquitin-protein ligase NHLRC1 protein (86-395aa)
-
免疫原種屬:Homo sapiens (Human)
-
標(biāo)記方式:Biotin
-
克隆類型:Polyclonal
-
抗體亞型:IgG
-
純化方式:>95%, Protein G purified
-
濃度:It differs from different batches. Please contact us to confirm it.
-
保存緩沖液:Preservative: 0.03% Proclin 300
Constituents: 50% Glycerol, 0.01M PBS, pH 7.4 -
產(chǎn)品提供形式:Liquid
-
應(yīng)用范圍:ELISA
-
Protocols:
-
儲存條件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
貨期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相關(guān)產(chǎn)品
靶點詳情
-
功能:E3 ubiquitin-protein ligase. Together with the phosphatase EPM2A/laforin, appears to be involved in the clearance of toxic polyglucosan and protein aggregates via multiple pathways. In complex with EPM2A/laforin and HSP70, suppresses the cellular toxicity of misfolded proteins by promoting their degradation through the ubiquitin-proteasome system (UPS). Ubiquitinates the glycogen-targeting protein phosphatase subunits PPP1R3C/PTG and PPP1R3D in a laforin-dependent manner and targets them for proteasome-dependent degradation, thus decreasing glycogen accumulation. Polyubiquitinates EPM2A/laforin and ubiquitinates AGL and targets them for proteasome-dependent degradation. Also promotes proteasome-independent protein degradation through the macroautophagy pathway.
-
基因功能參考文獻:
- Malin promotes its own degradation via auto-ubiquitination.Malin preferentially degrades the phosphatase-inactive laforin monomer. PMID: 26648032
- laforin/malin complex is able to interact with and ubiquitinate both PKM1 and PKM2 PMID: 26493215
- Lafora disease proteins laforin and malin negatively regulate the HIPK2-p53 cell death pathway. PMID: 26102034
- This study demonistrated that NHLRC1 mutations were detected in some case of Mild Lafora disease patients. PMID: 25270369
- Without functional laforin-malin complex assembled on polyglucosan bodies, polyglucosan is not degraded. PMID: 24068615
- Malin regulates the recruitment of mRNA-decapping enzyme 1A (Dcp1a) to processing bodies. PMID: 23131811
- Malin forms a functional complex with laforin. This complex promotes the ubiquitination of proteins involved in glycogen metabolism and misregulation of pathways involved in this process results in Lafora body formation. (Review) PMID: 22815132
- This study identified that NHLRC1 gene mutations leading to Lafora disease in six Turkish families. PMID: 22047982
- Our results indicate that malin regulates Wnt signaling pathway through the degradation of dishevelled2 and suggest possible deregulation of Wnt signaling in Lafora disease. PMID: 22223637
- Mutations in the NHL repeat containing 1 (NHLRC1) gene are described in association with a more benign clinical course and later age of death in an adolescent patient. PMID: 21555062
- Laforin and malin are defective in Lafora disease (LD), a neurodegenerative disorder associated with epileptic seizures PMID: 21652633
- malin(C46Y), malin(P69A), malin(D146N), and malin(L261P) mutants failed to downregulate the level of R5/PTG, a regulatory subunit of protein phosphatase 1 involved in glycogen synthesis. PMID: 21505799
- malin negatively regulates neuronatin and its loss of function in Lafora disease results in increased accumulation of neuronatin PMID: 21742036
- Malin is related to TRIM32 at both the phylogenetic and functional level. PMID: 21798009
- study described several novel mutations of EPM2A and NHLRC1 and brought additional data to genetic epidemiology of Lafora disease (LD); emphasized the high mutation rate in patients with classical LD as well as the high negativity rate of skin biopsy PMID: 20738377
- These results suggest that the modification introduced by the laforin-malin complex could affect the subcellular distribution of AMPK beta subunits. PMID: 20534808
- the co-chaperone carboxyl terminus of the Hsc70-interacting protein (CHIP) stabilizes malin by modulating the activity of Hsp70. PMID: 19892702
- Laforin and malin colocalize to the ER, suggesting they operate in a related pathway protecting against polyglucosan accumulation and epilepsy PMID: 12958597
- Genetic allelic heterogeneity is present in Lafora disease associated with mutations in EPM2B. Patients with mutations in EPM2A and EPM2B express similar clinical manifestation. PMID: 15781812
- Malin is an E3 ubiquitin ligase that ubiquitinates and promotes the degradation of laforin. PMID: 15930137
- Malin is an E3 ubiquitin ligase that binds glycogen synthase. PMID: 16115820
- Patients with NHLRC1 mutations have a slower rate of disease progression than those with EPM2A mutations. PMID: 16950819
- Defects in malin may lead to increased levels of misfolded and/or target proteins, which may eventually affect the physiological processes of the neuron, and likely to be the primary trigger in the physiopathology of lafora disease. PMID: 17337485
- Regulation of glycogen synthesis by the laforin-malin complex is modulated by the AMP-activated protein kinase complex pathway. PMID: 18029386
- malin ubiquitinates PTG in a laforin-dependent manner, both in vivo and in vitro, and targets PTG for proteasome-dependent degradation. These results suggest an additional mechanism, involving laforin and malin, in regulating glycogen metabolism PMID: 18070875
- The authors identified 14 Lafora epilepsy patients in the genetic isolate of tribal Oman. The authors show that in this homogeneous environment and gene pool, the same mutation, EPM2B-c.468-469delAG, results in highly uniform ages of onset and death. PMID: 18263761
- Results suggest that the altered subcellular localization of mutant proteins of the EPM2A and NHLRC1 genes could be one of the molecular bases of the Lafora disease phenotype. PMID: 18311786
- Laforin and malin interact with misfolded proteins and promote their degradation through the ubiquitin-proteasome system. PMID: 19036738
- phosphorylation of R5/PTG at Ser-8 by AMPK accelerates its laforin/malin-dependent ubiquitination and subsequent proteasomal degradation, which results in a decrease of its glycogenic activity. PMID: 19171932
- Results describe a novel homozygous single-nucleotide variant in the NHLRC1 gene in a Malian consanguineous family. PMID: 19322595
- laforin and malin play a role protecting cells from ER-stress, likely contributing to the elimination of unfolded proteins PMID: 19529779
- Meta-analysis of gene-disease association. (HuGE Navigator) PMID: 19267391
- The phosphatase laforin acts as a scaffold that allows malin to ubiquitinate protein targeting to glycogen (PTG). These results suggest an additional mechanism, involving laforin and malin, in regulating glycogen metabolism. PMID: 18070875
顯示更多
收起更多
-
相關(guān)疾病:Epilepsy, progressive myoclonic 2 (EPM2)
-
亞細(xì)胞定位:Endoplasmic reticulum. Nucleus. Note=Localizes at the endoplasmic reticulum and, to a lesser extent, in the nucleus.
-
組織特異性:Expressed in brain, cerebellum, spinal cord, medulla, heart, liver, skeletal muscle and pancreas.
-
數(shù)據(jù)庫鏈接:
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-